Chaperones and Mitochondrial Protein Import Machineries
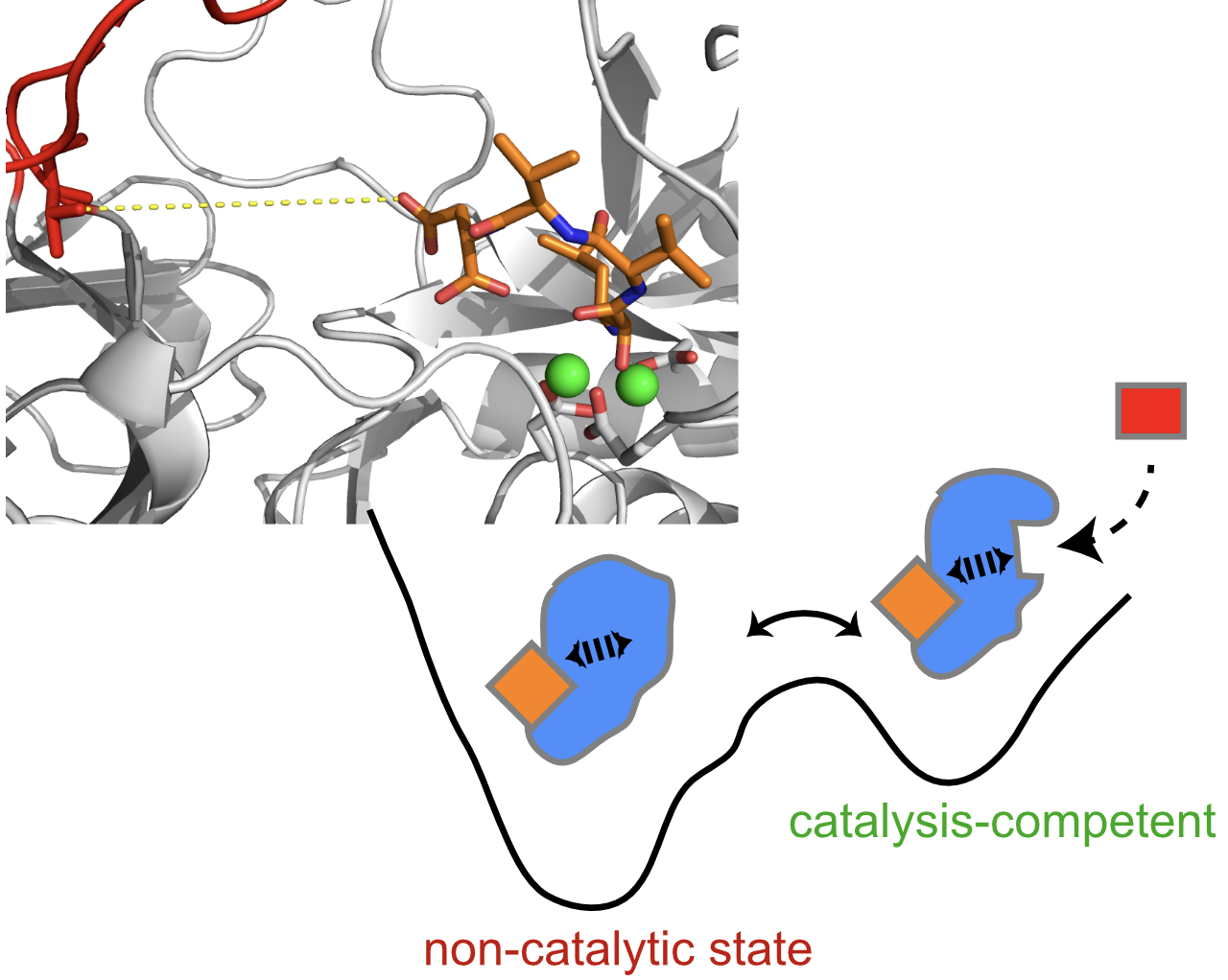
The viability of a cell critically depends on its capacity to securely hold, adeptly fold, and efficiently transport a multitude of proteins. Proteins are often notoriously prone to aggregation; many struggle to achieve their essential three-dimensional structure without assistance. Almost all mitochondrial proteins, for instance are synthesized in the cytosol, necessitating post-translational import into the organelle as ‘precursor proteins’. This arduous journey demands not only secure intracellular trafficking but also the delicate threading through minuscule membrane pores, and refolding within an aqueous environment or into one of the membranes. The main mitochondrial import pathways and machineries have been identified, and sometimes even the static 3D structures. However, we hardly know anything how the precursor proteins engage with the import machinery: the complexes are dynamic, their interactions are transient which makes their study difficult.
We delve into how chaperones, translocases, and insertases synergize to shepherd their delicate cargo to their destination. This investigational pursuit harnesses an array of structural biology techniques; specifically, using solution-NMR or MAS NMR—especially for membrane proteins—and determining structures through the power of cryo-EM. We peel back the dynamic layers of these extraordinary transient complexes, leveraging the full arsenal of advanced NMR dynamics experiments, SAXS, and MD techniques. Lastly, we perform experiments in live cells (functional assays and NMR) to bridge the in vitro revelations with tangible cellular behavior.
Read more:
- Structural Basis of Membrane Protein Chaperoning through the Mitochondrial Intermembrane Space
- Structural basis of client specificity in mitochondrial membrane-protein chaperones
- Preparing Chaperone–Client Protein Complexes for Biophysical and Structural Studies
- How do chaperones bind (partly) unfolded client proteins?
- Structural investigation of a chaperonin in action reveals how nucleotide binding regulates the functional cycle
- Architecture and assembly dynamics of the essential mitochondrial chaperone complex TIM9· 10· 12
Enzymes and Allostery
Enzymes are extraordinary catalysts that accelerate chemical reaction by many orders of magnitude. Furthermore, the activity of these remarkable biomolecules must be meticulously regulated and deftly adapted to the metabolic nuances of the cell. Allostery emerges as a pivotal force in this sophisticated regulation. The intrinsic dynamics of enzymes play a critical role, not only in the catalytic process itself but also as a crucial component intimately tied to allosteric regulation. Our research explores this fascinating interplay between enzyme function and dynamics. We are particularly driven to unveil the evolutionary forces that have sculpted enzyme functionality and allosteric behavior, revealing how these remarkable proteins adapt to extreme environmental conditions, such as soaring temperatures and the crushing depths of hydrostatic pressure reaching thousands of bar in the depths of the oceans.
To accomplish this, we employ a multi-faceted strategy incorporating solution- and MAS NMR, which we integrate with cryo-EM, crystallography, functional assays, bioinformatics, and molecular-dynamics simulations, among many others. Our use of NMR under high pressure (up to 3000 bar!) allows us to decipher the enigmatic mechanisms by which enzymes from deep-ocean organisms are activated through pressure. The systems we are often large – in the hundreds of kilodaltons range — , a challenge for NMR which we confront by utilizing specific isotope labeling (for both solution- and MAS NMR) and MAS NMR—a formidable technique with no inherent size limitations.
Read more:
- Disulfide-bond-induced structural frustration and dynamic disorder in a peroxiredoxin from MAS NMR
- Functional control of a 0.5 MDa TET aminopeptidase by a flexible loop revealed by MAS NMR
Excited states, disorder and folding
Proteins are characterized by a dynamic ensemble of conformational states. Conformational states with lifetimes as brief as milliseconds, which are only sparsely populated, often play pivotal roles in protein functionality. These states may manifest as locally disordered or unfolded regions, or as structured configurations that accommodate ligands or substrates. We use NMR spectroscopy to characterize these transient, “invisible” states.
Read more, e.g.:
More to come soon…
Fundamentals: Protein Motion
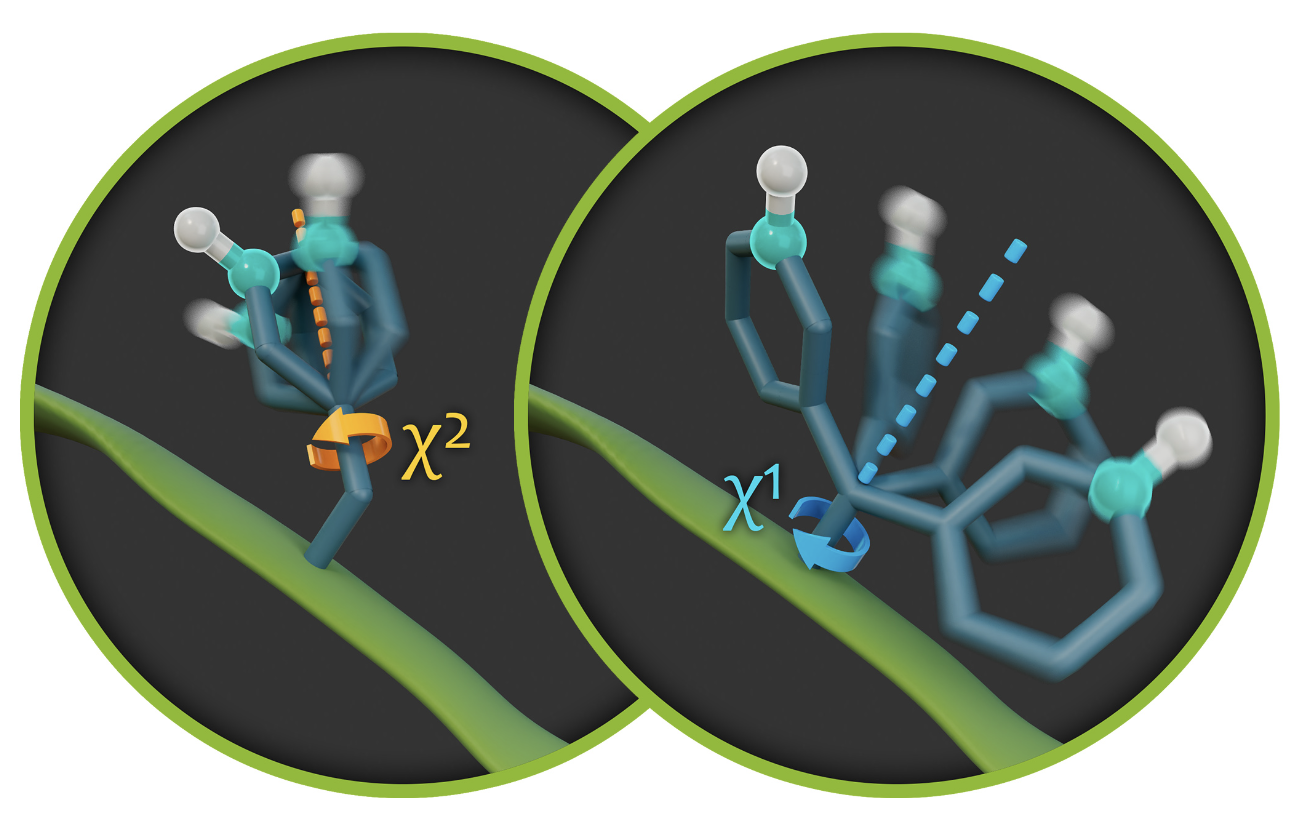
In addition to specific biological inquiries concerning chaperone activity or enzyme catalysis, we also ask fundamental questions related to dynamics of proteins. For instance, what mechanisms are required for the inversion of an aromatic ring? In what manner does the surrounding environment, or potentially the entire protein domain, facilitate such dynamic processes? How do the motions of proteins respond to the presence of binding partners or the dense packing of proteins within a crystal lattice? Furthermore, in what way does high pressure influence protein mobility, or in what manner are protein movements activated when a protein undergoes a transition from a deeply frozen state at 100 K to ambient room temperature? Enabled by and closely linked to methods we develop, we address such questions by NMR.
Read more:
- The Rigid Core and Flexible Surface of Amyloid Fibrils Probed by Magic‐Angle‐Spinning NMR Spectroscopy of Aromatic Residues
- Aromatic ring flips in differently packed ubiquitin protein crystals from MAS NMR and MD
- Aromatic Ring Dynamics, Thermal Activation, and Transient Conformations of a 468 kDa Enzyme by Specific 1H–13C Labeling and Fast Magic-Angle Spinning NMR
New Isotope Labeling Approaches
Nuclear Magnetic Resonance (NMR) is distinct within spectroscopic and structural-biology methodologies due to its capability to detect specific isotopes. By strategically positioning these isotopes, it becomes possible to focus on the most pertinent areas. Our research involves developing techniques aimed at the strategic placement of isotopes exclusively at amino acid types of interest, or at a limited number of precisely defined key locations. We formulate methods that integrate organic syntheses (conducted by our collaborators) with appropriate in-vivo or in-vitro incorporation of isotope-labeled compounds. These methodologies frequently enable novel spectroscopic techniques and may yield significant insights into, for example, the active sites of enzymes, where catalytic activity occurs.
Read more:
- Deuteration of proteins boosted by cell lysates: high-resolution amide and H magic-angle-spinning (MAS) NMR without the reprotonation bottleneck
- Aromatic Ring Dynamics, Thermal Activation, and Transient Conformations of a 468 kDa Enzyme by Specific 1H–13C Labeling and Fast Magic-Angle Spinning NMR
Pushing the Limits with new NMR Pulse Sequences
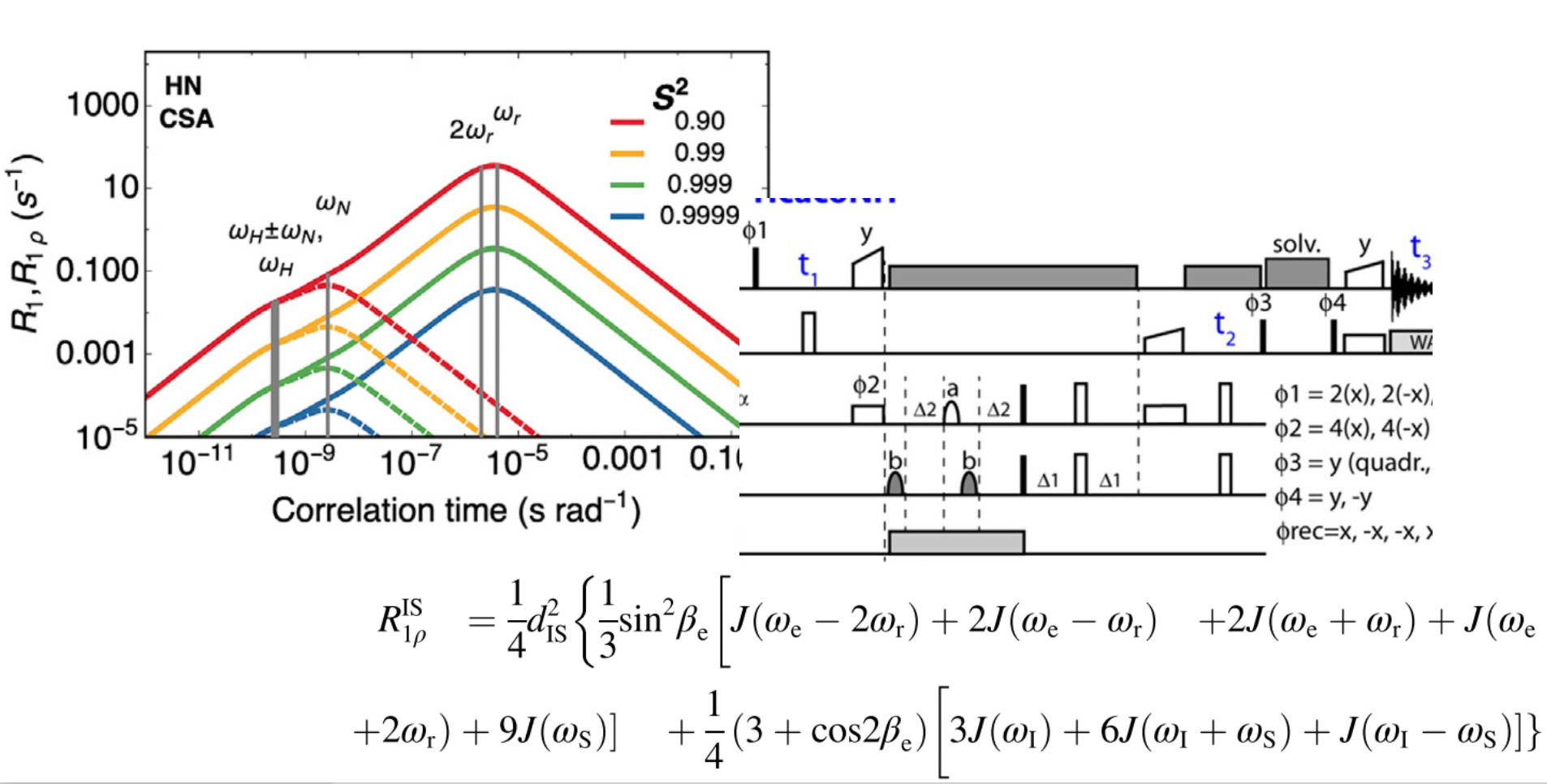
NMR is a formidable playground where quantum mechanics can be dealt with in a remarkably simple manner to extract new information about molecules. The improvement of methods has been instrumental and keeps an important role in NMR-based structural biology. Our group is particularly interested in methods allowing to see protein dynamics. For example, we have developed relaxation-based methods – such as the NERRD methodology to see microsecond motions. This activity of the group is often closely linked to computational methods and isotope labelling, and often driven by and applied to mechanistic questions arising in our biological projects. We also established a user-friendly library, to facilitate the use of many MAS NMR pulse sequences by the community, and develop tools to make MAS NMR faster and more straightforward, such as high-dimensional assignment experiments.
Moreover, we integrate NMR data with other data sources; in this context, we developed an approach for structure determination by combining NMR and cryo-EM data.
Read more:
- Integrated NMR and cryo-EM atomic-resolution structure determination of a half-megadalton enzyme complex
- Evaluating the motional timescales contributing to averaged anisotropic interactions in MAS solid-state NMR
- Protein dynamics detected by magic-angle spinning relaxation dispersion NMR
- Solid‐state NMR H–N–(C)–H and H–N–C–C 3D/4D correlation experiments for resonance assignment of large proteins
- Microsecond Protein Dynamics from Combined Bloch-McConnell and Near-Rotary-Resonance R1ρ Relaxation-Dispersion MAS NMR
Dive deeper into theory of MAS-NMR to probe molecular dynamics here. Dive even deeper here.
Machine Learning for NMR
NMR provides a wealth of data that contain a wealth of information about protein conformations and dynamics. The integration of machine learning techniques presents significant potential to extract this information with greater efficiency compared to current conventional methods. Furthermore, machine learning methodologies can enhance the stages of data acquisition and processing. Collaborating with the robust machine learning community at ISTA and internationally, we are advancing the development of machine learning approaches for NMR. Further details will be forthcoming.